Home Sleep Study Test in Newark
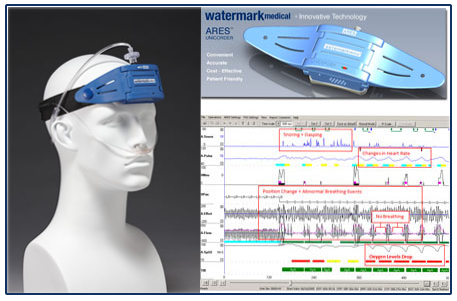
The Apnea Risk Evaluation System (ARES™) integrates physiological data acquired in-home with clinical history and anthropomorphic data to determine the presence and severity of Obstructive Sleep Apnea (OSA). The device is easy to operate for patients and for clinicians with a web-based portal for all study management and one-click physician interpretation. Apnea Risk Evaluation System (ARES)
Sleep Group Solutions is pleased to offer the innovative SleepMed ARES (formerly Watermark ARES) home sleep study and ARES screener to our clients. This device offers distinct advantages over other home sleep testing systems. The ARES screener is a peer-reviewed validated questionnaire that will generate a predicted risk level and severity prior to a patient ever taking home a sleep study. Based on the symptoms and predicted risk many are offered the ARES to take home for a full overnight (or multi-night) study.
ARES Home Sleep Test
A sleep-wearable wireless physiological recorder worn on the forehead that acquires and stores up to 3 nights of nocturnal data. ARES™measures blood oxygen saturation (SpO2) and pulse rate (reflectance pulse oximetry), airflow (by nasal cannula connected to a pressure transducer), snoring levels (calibrated acoustic microphone), head movement and head position (accelerometers). When worn in the home, the ARES™provides a better profile of the patient’s breathing during sleep in his/her normal environment. Audio and visual indicators notify the user when the ARES™ requires adjustment, thus increasing reliability of the device in the home. The small size of ARES™allows it to be comfortably worn in all sleep position.
Call Us Today
If you want to improve your smile or if you have any questions regarding our cosmetic dental services, please call our office at 510-744-9744. We'll be happy to answer any questions, inquiries, or concerns you may have.

